14 Management of Cowpea [Vigna unguiculata L. (Walp)] Germplasm Diversity in Senegal: A Crucial Asset for Breeding Programs
Amy Bodian, ISRA/CERAAS, Senegal
Awa Sarr, Cheikh Anta Diop University of Dakar, Senegal & ISRA/CERAAS, Senegal
Badara Guèye, IITA, Nigeria
Mouhamadou Moussa Diangar, ISRA/CNRA, Senegal
Tatiana Krasova Wade, IRD, Senegal
Demba Dramé, Cheikh Anta Diop University of Dakar & ISRA/CERAAS, Senegal
Ndiaga Cissé, ISRA/CERAAS, Senegal
Diaga Diouf, Cheikh Anta DIOP University of Dakar, Senegal
Abstract
Cowpea is a legume of nutritional, socioeconomic, and therapeutic importance in Senegal, as in other West African countries. However, biotic and abiotic constraints affect its production. To mitigate those constraints and enhance its productivity in Senegal, the management of cowpea genetic resources is a priority. Managing these resources involves extensive collection to cover maximum cowpea diversity, standard characterization, and sustainable conservation of the local germplasm. A review of the diversity management of the local cowpea germplasm and results from a study conducted between 2015 and 2021 are presented in this chapter. Many cowpea prospections and collections have been done since 1953, but they only covered a few cowpea growing areas.
Moreover, many collected accessions have been lost due to a lack of adequate conservation. This deprived cowpea breeding programs of great germplasm diversity. Therefore, in 2015, a cowpea collection was made, covering more production regions, including cultivated cowpea, hybrids, and wild accessions. The molecular characterization of this collection showed that the genetic diversity is higher in hybrids and wild accessions than in the cultivated species. These results will be used for a better valorization of cowpea genetic resources to overcome constraints encountered in the production of this crop.
Keywords: cowpea, local germplasm, diversity, breeding program, Senegal
Introduction
African agriculture faces major challenges to crop productivity in family farms. These challenges are in combination with climate change, which worsens soil degradation, drought, and heatwaves. Food demands are increasing due to the growing population and continual decrease in arable lands due to urbanization and industrialization. This requires a sustainable intensification of agricultural production based on resilient crops, such as cowpea, that can generate income and balance the diet of populations without degrading the ecosystem. Cowpea [Vigna unguiculata (L.) Walp] is mainly a self-pollinating plant. This important staple food crop, well-adapted to dry tropical regions and offering considerable economic, nutritional, and agronomic benefits (Agbicodoet al., 2009) in the tropical savanna areas of Africa, deserves special attention. Cowpea provides more than half of the vegetable proteins in the diet of populations in many developing countries, especially in sub-Saharan Africa (Aliyu &Wachap, 2014; Bhattarai et al., 2017; Boukaret al., 2016). It also contains a significant amount of minerals and vitamins. Moreover, cowpea has a short growing cycle, making it a reliable lean food for populations, especially during mid-season (around September in Senegal), when other crops have not reached maturity. It has economic importance because large quantities of its products (green pods, seeds, and haulms) can be marketed, providing a significant additional income to rural populations (both men and women). Cowpea is also processed into many popular food items such as couscous, thiakry, coffee, etc., representing another source of income for women processors. The crop is a legume adapted to drought conditions. It plays an important role as a source of nitrogen for other crops, such as cereal crops, especially in low soil fertility areas. Indeed, it fixes atmospheric nitrogen through its symbiotic associations with bacteria of the Bradyrhizobiumgenus, thus reducing the demand for nitrogen fertilizer and agricultural production costs (Asiwe et al., 2009; Dugje et al., 2008).
According to Ng and Maréchal (1985), the crop has been cultivated in Senegal for centuries. Séne (1966) hypothesized that local early maturity varieties were introduced from Nigeria for recession cultivation in the Senegal River valley in the northern part of the country. In contrast, the late maturity varieties were introduced from Mali. They were cultivated in association with millet in the wetter areas of Senegal and spread out all over the country, favored by trade and migration. Despite its importance, cowpea yields are relatively low in Africa, particularly in Senegal, at less than 700 kg/ha, according to a recent report released by the “Agence Nationale de la Statistique et de la Démographie” (ANSD, 2021). The low yields may be due to several abiotic (cold, heat, drought, low soil fertility, salinity, etc.), biotic (diseases, pests, parasitic plants, etc.), and socioeconomic constraints (Obilana, 1987; Singh & Sharma, 1996). Therefore, breeding programs in Senegal have been focused on developing high-yielding varieties adapted to different growing areas. Diverse, available, and accessible crop collections are crucial for the success of such crop improvement programs. According to the breeding objectives, new traits can be tapped from crop germplasm diversity, using advanced tools such as marker-assisted selection to speed up cowpea breeding.
Moreover, wild relatives can be an abundant source of interesting and exploitable genes for crop improvement (Feldman & Sears, 1981). However, lack of genetic diversity, information on a genetic marker, and knowledge of agronomic traits of interest constitute many limiting factors to cowpea breeding programs. This justified all cowpea prospection missions around the country that happened in the past, which were unfortunately not always sustainably managed and conserved for future use.
Herein, we review the work carried out in Senegal on the genetic diversity management of the local cowpea germplasm and present the results of a five-year study completed in 2021.
Cowpea Biodiversity Management in Senegal Before 2015
According to previous reports, cowpea biodiversity management started around 1950 in Podor (the northern part of Senegal) and allowed the selection of a local variety called 58-57. This variety is resistant to drought, reaches maturity 75 days after sowing, has a prostrate stand, and produces a significant number of seeds and haulms. In the same period, several prospecting missions were made in Senegal and beyond (in West Africa), which allowed the collection of 74 accessions (Séne, 1966). Between 1964 and 1967, germplasm evaluation work permitted the identification of cowpea varieties adapted to many production zones in Senegal.
In 1979, American varieties selected for drought and high temperatures tolerance were introduced in various Senegalese agro-ecological zones and compared with local varieties (Cissé et al., 1984). Most promising varieties showing the best adaptability were evaluated in larger trials before diffusion. Furthermore, they have been crossed with local varieties for transferring drought and heat tolerance and pests and disease resistance traits (Hall, 1982, 1984). Then, the breeding program prioritized crosses between the best local and introduced materials, focusing on the ones with promising drought tolerance and high yielding traits. Several cowpea varieties developed in Senegal (Ndiambour, Mougne, Bambey 21, Diongoma, Melakh, Mouride, Yacine, and Pakau) came from those crosses. In 1983, the Senegalese cowpea germplasm collection consisted of 535 accessions, including 347 early maturing lines (not photosensitive), 72 late (photosensitive), and 31 irradiated lines from different origins. The same year, new collections were carried out in the Center and the South of Senegal to complement the existing collection. A total of 85 samples were collected (Ndiaye, 1986).
In 2002 and 2014, the inventory of the national cowpea collection revealed that several accessions were lost due to conservation issues. In 2002, the number of accessions available in the “Centre National de RecherchesAgronomiques” (in Bambey) of “InstitutSénégalais de RecherchesAgricoles” (ISRA) was 247. This prompted a collection mission that resulted in the acquisition of 58 cowpea accessions collected in the Louga, Thies, and Diourbel regions of Senegal (North and Center-North) (Kouakouet al., 2007) and molecular and agro-morphological characterization of the germplasm. Unfortunately, in 2014, most of those accessions were lost again. This loss concerned all accessions collected in the main cowpea growing areas in the North and Center-North regions and the Center-South, the East, and the South of the country. Moreover, before 2015 no cowpea collection or prospection carried out in Senegal included wild cowpea relatives.
Cowpea Biodiversity Management in Senegal Between 2015 and 2021
Between 2015 and 2017, a new national cowpea collection was set up in the framework of a project funded by the West African Agricultural Productivity Program (WAAPP). The overall objective of this project was to contribute to the fight against poverty and food insecurity through better management of cowpea genetic resources. The aim was to set up, conserve and characterize a national cowpea collection for Senegal.
1. Germplasm Collection
Regarding the cultivated cowpea accessions, surveys and collections were carried out between September 2015 and March 2016 in the main cowpea growing regions of Senegal (Louga, Thies, Fatick, Diourbel) and additional regions such as Saint Louis (North), Sédhiou, Kolda (South) and Kédougou (Southeast). The villages covered during the collection were selected in consultation with rural development services (Division Régionale de Développement Rural, DRDR), allowing easier access to cowpea production villages. For optimal coverage, three departments were visited in each region, and four to six villages were visited in each department. An average of 10 farmers were interviewed per village, and seed samples were collected from the fields or granaries. Accessions of wild cowpea relatives were collected between November and December 2016 in six regions (Louga, Thiès, Diourbel, Fatick, Sédhiou, Kolda). Seven hundred thirty-one cultivated accessions and 82 wild relatives were collected across all the regions surveyed (Figure 1, Table 1).
Figure 1
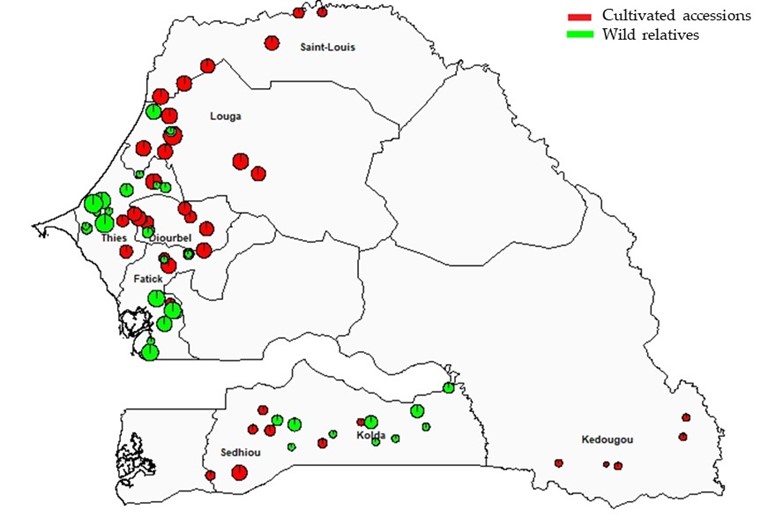
| Louga | Diourbel | Thiès | Fatick | Saint Louis | Sédhiou | Kédougou | Kolda | |
| Cultivated cowpea | 152 | 158 | 89 | 90 | 122 | 79 | 35 | 6 |
| Hybrids | 14 | 2 | 0 | 4 | 0 | 0 | 0 | 1 |
| Wild accessions | 0 | 2 | 22 | 21 | 0 | 3 | 0 | 13 |
| Total | 166 | 162 | 111 | 115 | 122 | 82 | 35 | 20 |
2. Genetic Characterization of the Collection
The collection of cultivated cowpea and wild relatives was sown in the screenhouse at the “Centre d’Etude Régional pour l’Amélioration de l’Adaptation à la Sécheresse” (CERAAS). Among the 731 cultivated accessions, only 671 germinated and were genotyped. However, all the 82 collected samples grew and were genotyped for the wild cowpea relatives. DNA has been extracted on fresh leaves of 21-day-old plants, and genotyping was done with fifteen (15) simple sequence repeat (SSR) markers (Table 2) selected from a previous screening of cowpea markers available at CERAAS. The genetic diversity of the national cowpea germplasm was then compared with that of 25 accessions from 5 countries (Nigeria, Niger, Burkina Faso, India, USA), kindly shared by the Genetic Resources Center (GRC) of the International Institute of Tropical Agriculture (IITA). The data were analyzed using R, GenAlex, STRUCTURE, and Darwin software to calculate genetic parameters, determine the genetic structure, and build dendrograms.
| Primer code | Sequence (5′-3′) | Tm (°C) |
| SSR6671-MF | CACGACGTTGTAAAACGACCAAACTTTGATATCGATCCTTG | 79.5 |
| SSR6671-R | GTTCTCTCATGCCATGATG | 59.2 |
| SSR6418-MF | CACGACGTTGTAAAACGACCAAATTCCCAAAGACATGTAA | 79.4 |
| SSR6418-R | CATGCAATGGCTAAAGGACA | 63.6 |
| SSR6777-MF | CACGACGTTGTAAAACGACGTTATCATATATGACAGTCTTTAATC | 75.2 |
| SSR6777-R | CATTTGATGCGGTTGATGGG | 68.3 |
| SSR6311-MF | CACGACGTTGTAAAACGACATGCCATTGTTGAGTTGCTTT | 81.5 |
| SSR6311R | AGGATGTTGTAGCAGGCTAATTG | 63.1 |
| SSR6800-MF | CACGCGTTGTAAAACGACTGACTCTTTCTCTCAAGTTA | 75.5 |
| SSR6800-R | GATGGGTTGTTGAAAATAAA | 56.5 |
| SSR6807-MF | CACGACGTTGTAAAACGACGAACTATTATACAATCATGCACGA | 79.2 |
| SSR6807-R | GTAGCTTACTTCAATGATTAG | 50.1 |
| SSR6289-MF | CACGACGTTGTAAAACGACCCCCCAAAGTTGATGAACAC | 82.5 |
| SSR6289-R | TTGATGGAGTTCGCATCTTCT | 63.7 |
| SSR6243-MF | CACGACGTTGTAAAACGACGTAGGGAGTTGGCCACGATA | 82.7 |
| SSR6243-R | CAACCGATGTAAAAAGTGGACA | 63.5 |
| SSR6304-MF | CACGACGTTGTAAAACGACCTCTCACATGCAATCCTAAATGGC | 82.9 |
| SSR6304-R | CTACGATAATGAGGATAACCATC | 58.1 |
| SSR6323-MF | CACGACGTTGTAAAACGACCAAAGGGTCATCAGGATTGG | 82.6 |
| SSR6323-R | TTTAAGCAGCCAAGCAGTTGT | 63.7 |
| MA113-MF | CACGACGTTGTAAAACGACTCGCACACAGATCCAACATT | 81.9 |
| MA113-R | CCTTATTTCTGGTGGGAGCA | 63.9 |
| SSR6241-MF | CACGACGTTGTAAAACGACGGGCAGAATGGAGATGAGAA | 83.3 |
| SSR6241-R | TTGATGGAGTTCGCATCTTCT | 63.7 |
| SSR6819-MF | CACGACGTTGTAAAACGCGCAACATGGAGGAAGATGCAAAG | 84.4 |
| SSR6819-R | CAAAAGAAATCATGATCTAACTTC | 57.6 |
| SSR6425-MF | CACGACGTTGTAAAACGACTGCTCAGTTCTGTGGTCCTG | 82.1 |
| SSR6425-R | TGGTTTATTCATCCAACATAGCA | 62.9 |
| SSR6217-MF | CACGACGTTGTAAAACGACGGGAGTGCTCCGGAAAGT | 83.6 |
| SSR6217-R | TTCCCTATGAACTGGGAGATCTAT | 62.7 |
| M13-tailed primer | CACGACGTTGTAAAACGAC |
2.1 Genetic Diversity of cultivated cowpea accessions
The genetic similarity values of the accessions collected from different regions ranged from 0.971 (Sédhiou and Diourbel) to 0.732 (Louga and Kolda) (Table 3). These results mean that the accessions from Sedhiou and Diourbel were genetically closer, while those from Louga and Kolda were the most distant ones. In general, the genetic diversity is low in the cultivated cowpea accessions. This low genetic diversity was found in many cowpea molecular studies using different technics (Asare et al., 2010; Badiane et al., 2012; Diouf & Hilu, 2005; Fatakun et al., 2018; Kouakou et al., 2007; Sarr et al., 2020; Sarr et al., 2021). This can be explained by the cowpea’s mode of reproduction, a self-pollinating plant.
| Thiès | Sédhiou | Kédougou | Saint Louis | Louga | Diourbel | Fatick | Kolda | |
| 1.000 | Thiès | |||||||
| 0.916 | 1.000 | Sédhiou | ||||||
| 0.860 | 0.956 | 1.000 | Kédougou | |||||
| 0.851 | 0.907 | 0.826 | 1.000 | Saint Louis | ||||
| 0.912 | 0.945 | 0.861 | 0.882 | 1.000 | Louga | |||
| 0.866 | 0.971 | 0.917 | 0.897 | 0.948 | 1.000 | Diourbel | ||
| 0.954 | 0.912 | 0.830 | 0.891 | 0.922 | 0.906 | 1.000 | Fatick | |
| 0.762 | 0.782 | 0.768 | 0.745 | 0.732 | 0.736 | 0.770 | 1.000 | Kolda |
The intra-regional variability was greater (79%) than the inter-regional variability (14%) (Table 4). These results agree with those of Sarret al.(2020) (75% versus 11%). The high intra-regional diversity could be linked to many different accessions in each region. In contrast, the low genetic diversity between regions could be partly explained by the distribution of the same cowpea seeds (same varieties found everywhere) in all the regions through donations, seed companies, or agricultural extension services.
| Source of variation | Variations (%) | Fst |
| Among Populations | 14 | 0.141*** |
| Among Individuals | 79 | |
| Within Individual | 7 | |
| Total | 100 |
Fst: Genetic differentiation Index; ***p ≤ 0.001
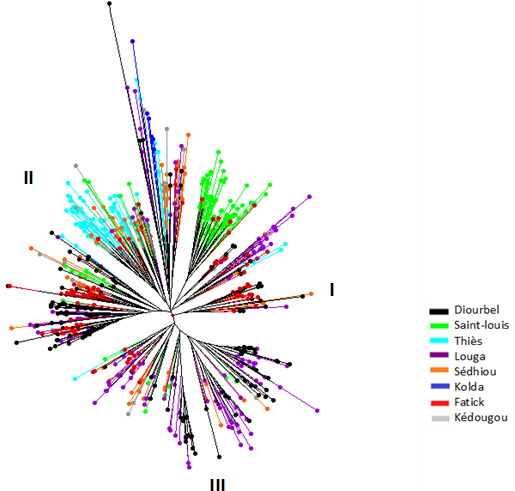
The dendrogram showed three main genetic groups and several subgroups. The genetic structure of collected accessions was independent of geographical origin. However, cultivated cowpea accessions from floodplain recession areas were all in a single subgroup. This can be explained by the low gene flow between accessions cultivated in floodplain recession areas and the rainfed areas. They are cultivated in different growing systems and flower at different times (Figure 2).
Figure 2
2.2 Genetic Diversity of Wild Relatives Accessions (Hybrids and Wilds)
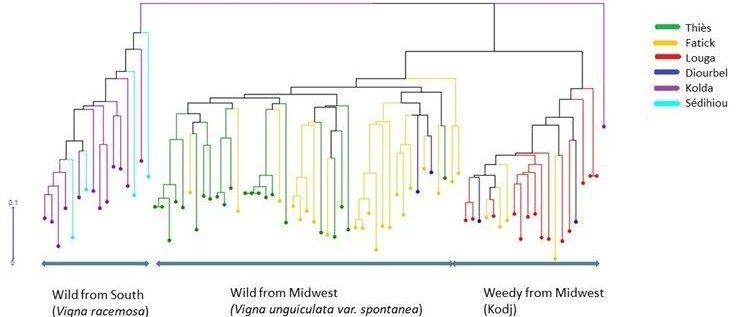
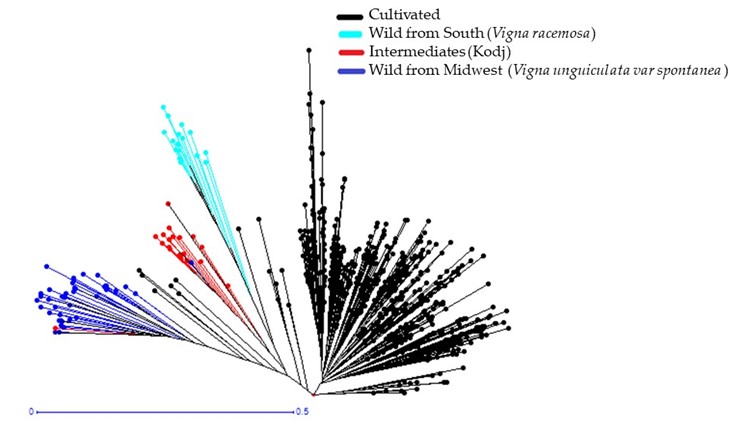
The dendrogram showed that hybrids and wilds were divided into three groups according to their type and origin (Figure 3). The first group contained the wild accessions from southern Senegal (Kolda and Sédhiou). The second group contained all the accessions from the Midwest (Louga, Thiès, Diourbel, and Fatick). This group was subdivided into two subgroups. The first subgroup comprises the 45 wild accessions described as Vigna unguiculata var. spontanea from Thiès, Fatick, and Diourbel. The second is the 20 accessions identified as hybrids (weedy) called “Kodj” in the local name from Louga, Fatick, and Diourbel. Usually, these hybrids germinate spontaneously in farmers’ fields. After harvest, farmers did not mix them with their seeds. They sort the seed of the hybrids and throw them away because they take a longer time to cook as their seed coat is harder than that of the cultivated. Moreover, the seed coat of the hybrids is smoother and the size smaller than that of the cultivated cowpea but larger than that of the wild species. The third group included only the one hybrid from Kolda.
Figure 3
The genetic differentiation index (Fst) was high, reaching 0.169 between wild accessions from Midwest (Vigna unguiculata var. spontanea) and hybrids (weedy), 0.234 between those from Midwest and the South (Vigna racemosa), and 0.353 between hybrids and southern wild accessions (Table 5). Wilds from the South belong to the Vigna racemosa species. This species is different from Vigna unguiculata var spontanea from the Midwest. The speciation and the geographical difference could have limited the gene flow between these two species and explained the high genetic differentiation index.
| Hybrids | Wild from Midwest | Wild from South | |
| Hybrids | 0.000 | ||
| Wild from Midwest | 0.169 | 0.000 | |
| Wild from South | 0.353 | 0.234 | 0.000 |
***p-value<0.001
2.3 Genetic Diversity of the Overall Collection (Cultivated, Hybrids, and Wilds)
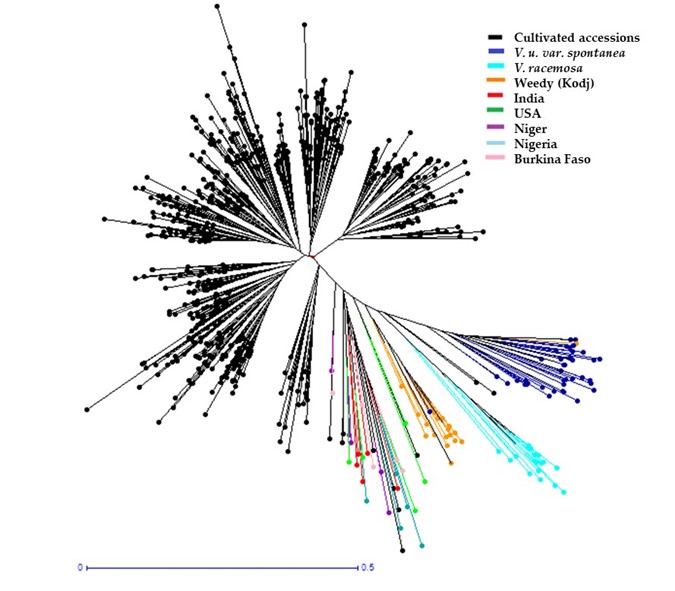
Cluster analysis of the entire Senegalese cowpea collection (i.e., cultivated, hybrids, and wild accessions) showed two genetic groups with 1.3% admixture. The first one contained the hybrids and the wild accessions (Vigna unguiculata var. spontanea and Vigna racemosa). The second group included the cultivated accessions. The principal coordinate analysis (PCoA) showed the two distinct genetic groups (Figure 4). The dendrogram of the cultivated cowpea, hybrids, and wild accessions’ genetic dissimilarity showed three major groups. The first and second were homogeneous and included only the cultivated accessions. The third group included mainly the wild accessions and the hybrids (Figure 5). The fact that hybrids were clustered with wilds could be explained by their genetic proximity revealed by the 15 SSSR used.
Figure 4a
Figure 4b
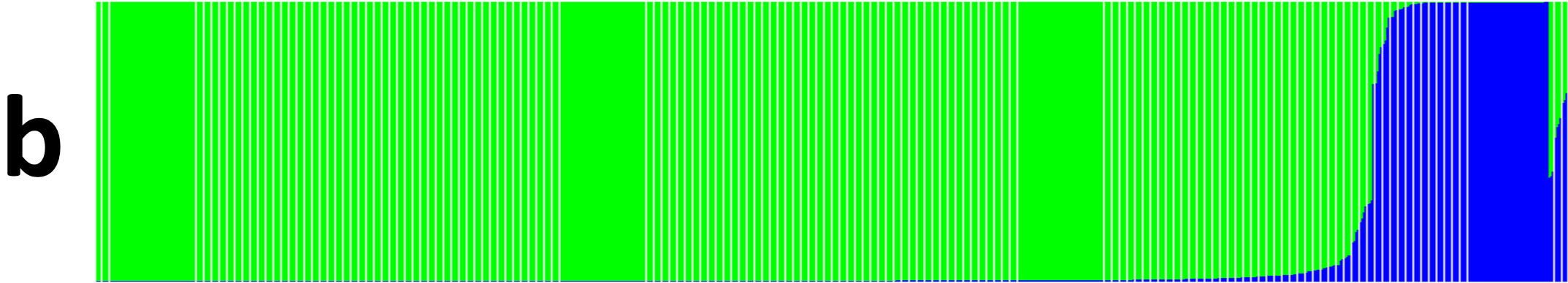
Figure 4c
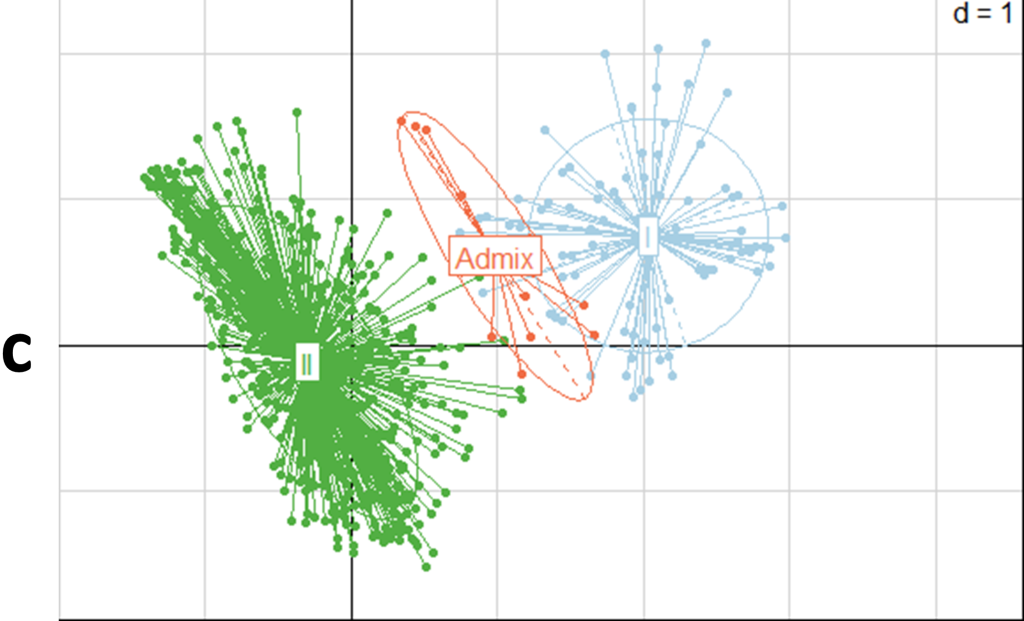
Figure 5
All the values of the diversity indices calculated for the two populations, the mean number of alleles (Na), expected heterozygosity (He), and fixation index (F), based on the allelic frequencies for the 15 loci tested, were higher for the hybrids and wilds than for the cultivated cowpea. The genetic differentiation index value (Fst) was 0.229, with the number of migrants (Nm) equal to 2.557 (Table 6).
| Parameters | Group II | Group I |
| Sample size (N) | 662 | 83 |
| Mean number of alleles (Na) | 5.4 | 6.267 |
| Expected Heterozygosity (He) | 0.382 | 0.552 |
| Fixation Index (F) | 0.834 | 0.869 |
| Genetic differentiation Index (Fst) | 0.229*** | |
| Number of migrants (Nm) | 2.557 | |
***p-value < 0.001
2.4 Comparison of the Genetic Diversity of the Senegalese Cowpea Germplasm with the Reference Cowpea Collection from IITA
The average genetic diversity observed for the national cowpea germplasm was lower (0.457) than the average diversity of accessions from Nigeria (0.508). At the same time, it was comparable to those obtained for the accessions from India (0.430). These values were higher when compared with the values obtained for accessions from Niger (0.328), Burkina Faso (0.380), and the USA (0.385).
The highest genetic differentiation index was observed between Senegal and USA (Fst = 0.253, p-value = 0.002). So, the Senegalese accessions and those from the USA are the most distant. Important differentiations were also found between Senegal and India (Fst = 0.155), Senegal and Niger (Fst = 0.183), and Senegal and Burkina Faso (Fst = 0.147). The genetic differentiation index (Fst = 0.115) is moderate between Senegal and Nigeria. The ancient introduction could explain this in Senegal of accessions from Nigeria (Sène, 1966). The lowest Fst was noted between Indian and Nigerian accessions (0,101); it could mean that they are genetically closer (Table 7). This study represents accessions from Niger, Nigeria, Burkina Faso, India, and the USA by five samples each. Therefore, the comparison made in this part is only valid for this small number of samples.
| Senegal | USA | India | Niger | Nigeria | Burkina Faso | |
| 0.000 | Senegal | |||||
| 0.253 | 0.000 | USA | ||||
| 0.155 | 0.149 | 0.000 | India | |||
| 0.183 | 0.253 | 0.198 | 0.000 | Niger | ||
| 0.115 | 0.171 | 0.101 | 0.133 | 0.000 | Nigeria | |
| 0.147 | 0.209 | 0.188 | 0.119 | 0.117 | 0.000 | Burkina Faso |
Figure 6
Conclusion and Perspectives
Thanks to the important collection and characterization efforts, ISRA has assembled a wide collection of cowpea genetic resources that is representative of the local germplasm and combines old and new collections. The old collection consisted of only cultivated cowpea, while the new one consisted of cultivated hybrids and wild accessions. Besides, this new collection has been genotyped. Cowpea wild relatives, having higher genetic diversity than cultivated cowpea with moderate differentiation, can provide important genes for tolerance or resistance to various stresses that hinder production. Similarly, the five accessions from the USA that were most distant from those from Senegal could provide new genes for crop improvement. The good management of the local cowpea germplasm could improve crop productivity in Senegal in the context of climate change. This improvement will positively impact the livelihoods of both male and female farmers, both nutritionally and economically.
Following this work of collecting and characterizing local Senegalese germplasm, it would be interesting to evaluate the wild relatives for traits related to drought tolerance, Striga, and Macrophomina resistance which are major constraints to cowpea cultivation in Senegal.The most promising accessions could then be used to make crosses with cultivated cowpea to identify QTLs associated with the traits of interest and yield components.
Acknowledgements
We thank the West African Agricultural Productivity Program (WAAPP) for funding the study and the International Institute of Tropical Agriculture (IITA) for providing the global accession from Niger, Nigeria, Burkina Faso, India, and the USA. We also thank AblayeNgom and Rémy Pasquet for their help in identifying cowpea species.
References
Agbicodo, E. M., Fatokun, C. A., Muranaka S., Visser, R. G. F., & Linden Van Der, C. G. (2009). Breeding drought tolerant cowpea: Constraints, accomplishments, and future prospects. Euphytica, 167, 353–370.
Agence Nationale de la Statistique et de la Démographie (ANSD). (2021). Bulletin mensuel des statistiques économiques et financières d’Avril, p. 110.
Aliyu, B., & Wachap, E. (2014). Vegetable cowpea as a source of cheap protein and an environmentally friendly crop for urban cities. Emerging Infectious Diseases,181, 301–312.
Asare, A. T., Gowda, B. S., Galyuon, I. K. A., Aboagye, L. L., Takrama, J. F., & Timko, M. P. (2010). Assessment of the genetic diversity in cowpea (Vigna unguiculata L. Walp.) germplasm from Ghana using simple sequence repeat markers. Plant Genet Resour, 8, 142–150. https://doi.org/10.1017/S1479262110000092
Asiwe, J. A. N., Balane, A., & Dacora, F. D. (2009). Evaluation of cowpea breeding lines for nitrogen fixation at ARC-Grain Crop Institute, Potchefstroom, South Africa. The 16th International Congress on Biological Nitrogen Fixation, Montana, USA. 14–19.
Badiane, F. A., Gowda, B. S., Cissé, N., Diouf, D., Sadio, O., & Timko, M. P. (2012). Genetic relationship of cowpea (Vigna unguiculata) varieties from Senegal based on SSR markers. Genetics and Molecular Research, 11, 292–304.
Bhattarai, P., Thomas, A. K., Cosacak, M. I., Papadimitriou, C., Mashkaryan, V., Zhang, Y. & Kizil, C. (2017). Modeling amyloid-β42 toxicity and neurodegeneration in adult zebrafish brain. Journal of Visualized Experiments: JoVE, Oct 2017(128).
Boukar, O., Fatokun, C. A., Huynh, B. L., Roberts, P. A., & Close, T. J. (2016). Genomic tools in cowpea breeding programs: Status and perspectives. Front. Plant Sci, 7(757). https://doi.org/10.3389/fpls.2016.00757
Cissé, N., 2015. Fiches variétales niébé et sorgho. Fiches techniques ISRA, 8(4).
Cissé, N., Thiaw, S., & et Sène, A. (1984). Projet CRSP/NIEBE – Essais variétaux Dot. Ronéo – ISRA/Bambey, p. 7.
Diouf, D. & Hilu, K. W. (2005). Microsatellites and RAPD markers to study genetic relationships among cowpea breeding lines and local varieties in Senegal. Genetic Resources and Crop Evolution, 52,1057–1067.
Diouf, M., Diallo, S., Badiane, F. A., Diack, O., & Diouf, D. (2021). Development of new cowpea (Vigna unguiculata) mutant genotypes, analysis of their agromorphological variation, genetic diversity and population structure. BIOCELL. https://doi.org/10.32604/biocell.2021.013706.
Dugje, I. Y., Ekeleme, F., Kamara, A.Y., Omoigui, L.O., Tegbaru, A., Teli, I. A., & Onyibe, J. E. (2008). Guide to safe and effective use of pesticides for crop production. International Institute of Tropical Agriculture, Jan. 2008. https://doi.org/10.13140/2.1.2721.8566
Fatokun, C., Girma, G., Abberton, M., Gedil, M., Unachukwu, N., Oyatomi, O., Yusuf, M., Rabbi, I., & Boukar, O. (2018). Genetic diversity and population structure of a mini-core subset from the world cowpea (Vigna unguiculata (L.) Walp.) germplasm collection. Scientific Reports, 8(16035). https://doi.org/10.1038/s41598-018-34555-9
Feldman, M., & Sears, E. R. (1981). The wild gene resources of wheat. Sci Amer, 2441,102‒112.
Hall, E. A. (1982). Report on Research at the University of California, Riverside in 1982 and proposa1 research for 1983. Manuscript – Riverside CA – UCR, Department of Botany and Plant Sciences, 12.
Hall, E. A. (1984). Summary Report Research at the University of California, Riverside in 1983. Manuscript – Riverside, CA – UCR Department of Botany and Plant Sciences, 5.
Kouakou, C. K., Roy-Macauley, H., Guèye, M. C., Otto, M. C., Rami, J. F., & et Cissé, N. (2007). Diversité génétique des variétés traditionnelles de niébé [Vigna unguiculata (L.) Walp.] au Sénégal : étude préliminaire. Plant Genet Resour News, 1(152), 33–44.
Morita, R., Kusaba, M., Iida, S., Yamaguchi, H., Nishio, T., & Nishimura, M. (2009). Molecular characterization of mutations induced by gamma irradiation in rice. Genes & Genetic Systems, 84, 361–370. https://doi.org/10.1266/ggs.84.361
Naito, K., Kusaba, M., Shikazono, N., Takano, T., Tanaka, A., Tanisaka, T., & Nishimura, M. (2005). Transmissible and nontransmissible mutations induced by irradiating Arabidopsis thaliana pollen with γ-rays and carbon ions. Genetics, 169, 881–889. https://doi.org/10.1534/genetics.104.033654
Ndiaye, M. (1986). Bilan de 30 ans de recherche sur le niébé au Sénégal. Rapport ISRA.
Ng, N. Q., & Marechal, R. (1985). Cowpea taxonomy, origin and germplasm. In: Cowpea research, production and utilization. Singh, S. R. and Rachie, K.O. (eds.), John Wiley and Sons, Chichester, UK, pp 11-21.
Obilana, A. T. (1987). Breeding cowpea for Siriga resistance. In L. J. Musselman (Ed.), Parasitic weeds in agriculture (pp. 243-253). CRC-Press, Inc.
Sarr, A., Bodian, A., Gbedevi, K. M., Ndoye Ndir, K., Oyatomi, A. O., Gueye, B., Foncéka, D., Diop, E. A. M. C., Diop, B. M., Cissé, N., & Diouf, D. (2020). Genetic diversity and population structure analyses of wild relatives and cultivated cowpea (Vigna unguiculata (L.) Walp) from Senegal using simple sequence repeat markers. Plant Molecular Biology Reporter. https://doi.org/10.1007/s11105-020-01232-z
Sarr, A., Bodian, A, Ouattara, B., Diangar, M., M., Sall, M. N., Diop, E. A. M. C., Diouf, D., & Cissé, N. (2021). Population structure and diversity among improved cowpea varieties from Senegal based on microsatellite markers. Global Journal of Molecular Biology, 3(7). https://doi.org/10.28933/gjmb-2020-12-2305
Séne, D. (1966). Inventaire des principales variétés de niébé (Vigna unguiculata Walpers) cultivées au Sénégal. L’Agronomie Tropicale, 927–933.
Singh, B. B., & Sharma, B. (1996). Restructuring cowpea for higher yield. Indian Journal of Genetics and Plant Breeding 56, 389–405.

